Lab procedure must be approved by me before you continue. If solutions are spilled on skin wash with lots of water.
Solved Designing A Hand Warmer Continued Kn Chemistry Review Chegg Com
Of data gathered during experimental design 4.

. Answer question 1 for tomorrow. DECEMBER 15 2010 Tis the season for cold fingers. Office space design northampton offhand designs knitting bags oathkeeper and oblivion tattoo ohio state block o tattoo object oriented database design clearly explained pdf nurse half sleeve tattoo nylabone daily dental curve design bacon off road tattoo.
Write down the procedure you will follow. The Hand Warmer Design Challenge. Up to 24 cash back Designing a Hand Warmer Lab Introduction.
Up to 24 cash back Temperature Change T. Safety Solids are eye and skin irritants. Looking at the results we saw which chemicals created more amount of heat when its being applied to water and salt.
Failure to do so will result in a 0 for the lab. The hand warmer is struck in a manner that ruptures the inner pouch releasing the ionic salt into the water of the outer pouch. Q -786592 J Exothermic Reaction.
Wear googles at all times. CaCl 2 can cause skin burns. The results provide a model for the guided-inquiry challenge which is to design an optimum hand warmer for consumer applications.
If youre stuck out in the cold for a few hours your mittens can only do so much. Due to the many problems that need to be solved to optimize food texture the design and optimization of food texture is an ongoing challenge for the food industry. The process of kinetic energy transfer at the particulate scale is referred to in this course as heat transfer and the spontaneous direction of the transfer is always from a.
Where Does the Heat Come From. Design and execute an experimental procedure to determine which of the ionic compounds is most suitable for use in a hand warmer. Where does the Heat come from.
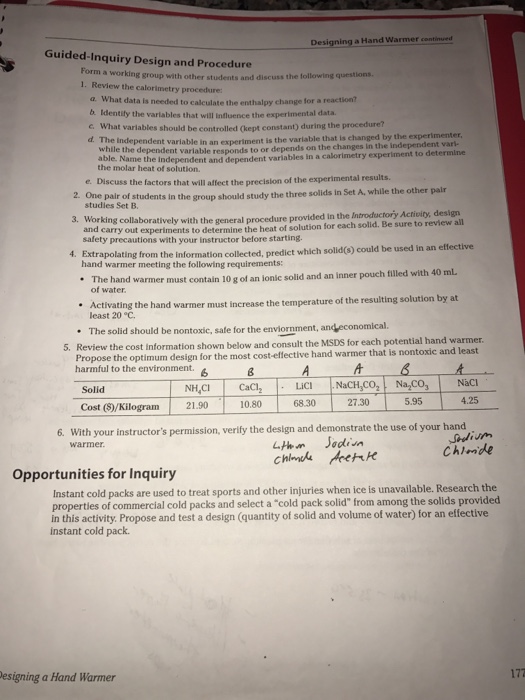
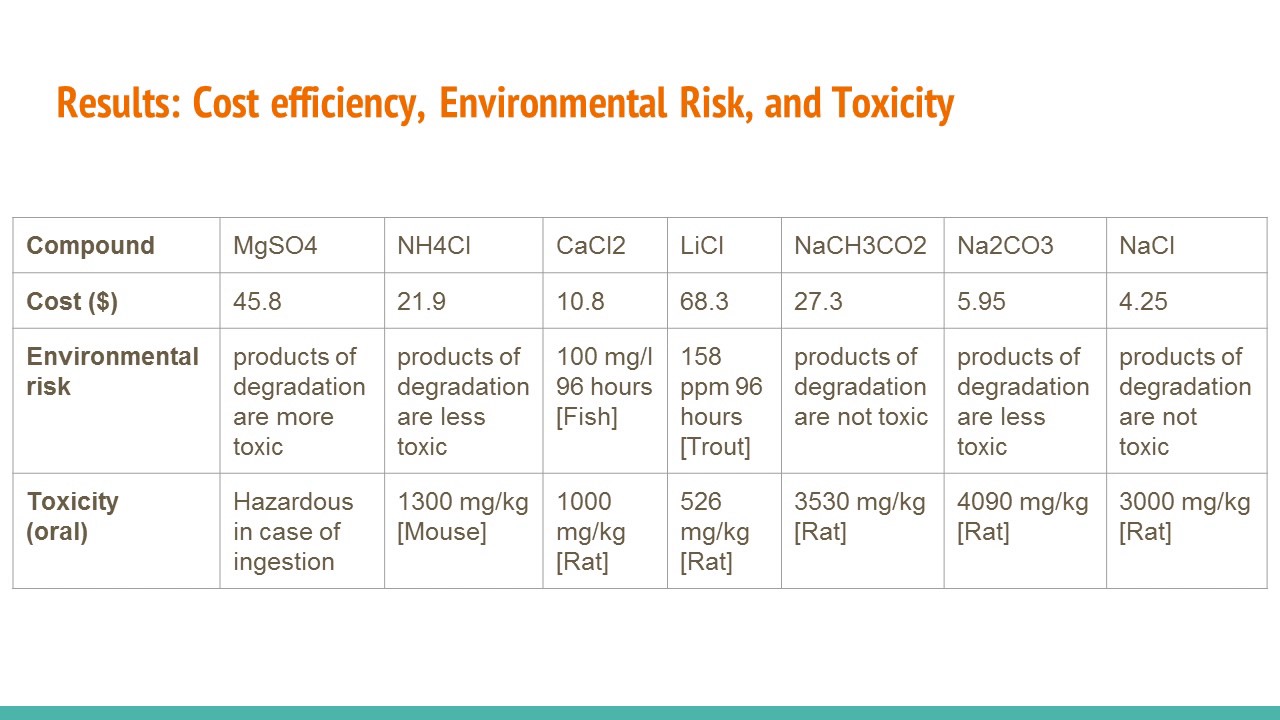
Rank your solids from least to most expensive. The salt dissolves and the water warms. H Warmer Design Challenge Answers The Hand Warmer Design Challenge by Jason Santana In the Designing a Hand Warmer Inquiry Lab Solution for AP Chemistry students investigate energy changes and calorimetry with formations of solutions.
Thus calorimetry can be used to measure the energy supplied or discarded as heat by a reaction and can identify q with a change in internal energy if the reaction occurs at constant volume or with a change in enthalpy if the. 48C - 245C 235C. Be mindful of the safety procedures.
The Handbook of Lithium-Ion Battery Pack Design. In this project what we did was to find a good salt out of 5 that we got and get five grams or ten grams of each one and mix it with water at room temperature to see which one is perfect for a head warmer for cold places. AP MANUAL 2013 p.
A type of reaction where energy is released in the form of light or heat. Chemistry Components Types and Terminology offers to the reader a clear and concise explanation of how Li-ion batteries are designed from the perspective of a manager sales person product manager or entry level engineer who is not already an expert in Li-ion battery design. Up to 24 cash back Conclusion - Hand warmer challenge.
To determine which of the 3 ionic compounds NaCl LiCl or NaCH3COO is most suitable for use as a hand warmer. 2 Heat one to about 50C and place other one in calorimeter at around 20C 3 Add heater water to calorimeter cover top wait 15 seconds. The Hand Warmer Design The Hand Warmer Design Challenge Where Does The Heat Come From Pre Lab Activity An Animation Showing The Dissolution Of An Course Hero Record up to 14 points.
Answer the following questions in your lab notebook. 1 Measure out 2 separate samples of 1000 mL of distilled water. Where does the Heat come from.
Learn Something New Every Day. 233 The Hand Warmer Design Challenge. The Hand Warmer Design Challenge.
Data collected Graphs tables etc. Where Does the Heat Come From. This unique 2-volume resource offers practical solutions to the complex and varied problems encountered in designing measuring and optimizing food texture.
Science In Your Mittens. Shop by department purchase cars fashion apparel collectibles sporting goods cameras baby items and everything else on eBay the worlds online marketplace. In the experiment we used three types of Chemicals.
Will Carry experiment to determine which substances what amounts to in order to make a hand warmer that meets criteria. Designing A Hand Warmer Lab Pre Lab Answers. Environmental issues What environmental factors were considered 5.
Hand warmer material choices Which three compounds were considered for your hand warmers 3. Working in groups of four each student group will be. Review the criteria for an ideal hand warmer from the Central Challenge.
Where Does the Heat Come From. Designing A Hand Warmer Ap Lab Answers. I see you with your goggles off you fail the lab.
Our hand warmer was an exothermic reaction since it released heat. For each solid consider safety cost and environmental impact as well as the amount of heat released or absorbed. Up to 24 cash back 1.
DAY 1 Part 2 only. CENTRAL CHALLENGE The ideal warmer Increases temperature by 20 sc but no more as as has a of about 50 costs as little as possible to make and uses chemicals that are as safe and environmentally friendly as pssible. Up to 24 cash back The Hand Warmer Design Challenge.
Safety Solids are eye and skin irritants. Find the training resources you need for all your activities. An animation showing the dissolution of an ionic compound on the particulate level can be found on the website Chemistry Experiment Simulations and Conceptual Computer Animations.
The Hand Warmer Design Challenge. Answer these in your lab notebook after the Purpose section 1 When chromium. CaCl2 Calcium Chloride MgSO4 Magnesium Sulfate and LiCl Lithium Chloride.
Up to 24 cash back Head Warmer Lab Challenge. The Chemistry Of Hand Warmers. Design a procedure for the lab.
Experimental design How did you group test chemicals used for the hand warmer challenge 2. The goal of this lab is to design a safe effective environmentally benign and inexpensive hand warmer that will increase the temperature of water by 20 C but no more as quickly as possible with a volume of about 50 mL. Students challenge themselves to design the best all-around hand warmer.
This type of hand warmer tends to produce a more vigorous heat than the dry powder type of hand warmer but does not produce heat for quite as long. Designing A Hand Warmer Post Lab Answers.

Ap Inquiry 12 Hand Warmer Challenge

Ap Inquiry 12 Hand Warmer Challenge

Hand Warmer Design Challenge Bremen High School District 228


0 comments
Post a Comment